As carbon dioxide continues to build up in the Earth’s atmosphere, research teams around the world have spent years seeking ways to remove the gas efficiently from the air. Meanwhile, the world’s number one “sink” for carbon dioxide from the atmosphere is the ocean, which soaks up some 30 to 40 percent of all of the gas produced by human activities.
Recently, the possibility of removing carbon dioxide directly from ocean water has emerged as another promising possibility for mitigating CO2 emissions, one that could potentially someday even lead to overall net negative emissions. But, like air capture systems, the idea has not yet led to any widespread use, though there are a few companies attempting to enter this area.
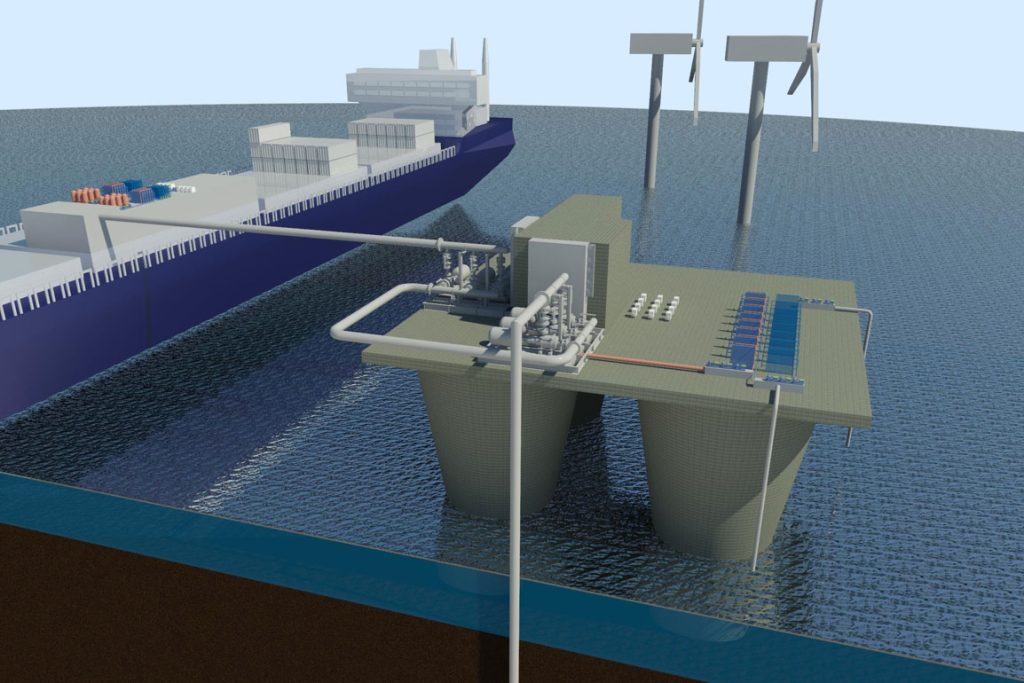
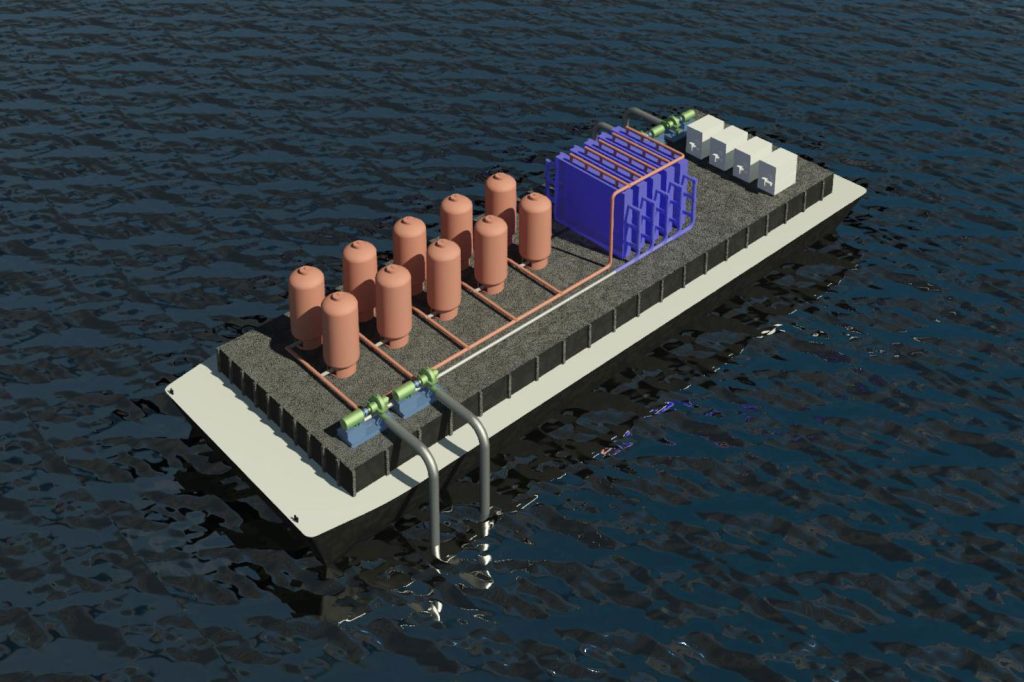
Now, a team of researchers at MIT says they may have found the key to a truly efficient and inexpensive removal mechanism. The findings were reported this week in the journal Energy and Environmental Science, in a paper by MIT professors T. Alan Hatton and Kripa Varanasi, postdoc Seoni Kim, and graduate students Michael Nitzsche, Simon Rufer, and Jack Lake.
The team came up with a reversible process consisting of membrane-free electrochemical cells. Reactive electrodes are used to release protons to the seawater fed to the cells, driving the release of the dissolved carbon dioxide from the water. The process is cyclic: It first acidifies the water to convert dissolved inorganic bicarbonates to molecular carbon dioxide, which is collected as a gas under vacuum. Then, the water is fed to a second set of cells with a reversed voltage, to recover the protons and turn the acidic water back to alkaline before releasing it back to the sea. Periodically, the roles of the two cells are reversed once one set of electrodes is depleted of protons (during acidification) and the other has been regenerated during alkalization.
Click here for more info.